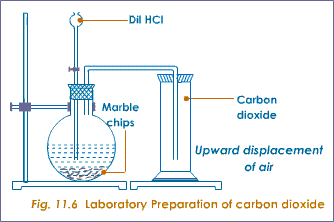
The rate of this reaction can be changed by changing the size of the marble chips.
Marble chips and hydrochloric acid.
This is the word equation for my investigation calcium hydrochloric calcium carbon water.
An investigation into how changing one variable influences the rate of reaction between marble chips and dilute hydrochloric acid planning section when dilute hydrochloric acid reacts with marble chips the following reactions occurs.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Secondly weight out 3 grams of marble chips for each concentration 3.
With the equation caco3 2hcl cacl2 h2o co2 hypotheses a reaction occurs when particles collide.
The temperature increase can be detected using a thermometer.
Figure 9 shows the apparatus the student used.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
Place 40cm 3 of hydrochloric acid in an conical flask add 20g of marble chips place a loose plug of cotton wool in the neck of the flask to prevent acid spray escaping place the whole apparatus on a balance and find its mass.
You then fill a bowl with water along with a boiling tube and straight after attach the delivery tube at the end of the boiling tube 4.
The energy is usually transferred as heat energy causing the reaction mixture and its surroundings to become hotter.
These are reactions that transfer energy to the surroundings.
We will be investigating what changes the rate of reaction.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Pour the acid into the conical flask and add the marble chips 5.
Image carbonate acid chloride dioxide.
First you measure out 25cm3 of hydrochloric acid 2.
Describe how you could investigate.
I am investigating how the rate of reaction between marble chips and hydrochloric acid is altered when the concentration is changed.
This reaction between marble chips calcium carbonate and hydrochloric acid is an exothermic reaction.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
Caco3 2hcl h2o co2 this is the reaction we will be investigating.
This process is based on random particle movement.
A student investigated the rate of reaction between marble chips and hydrochloric acid hcl.